
The magnesium displaces the copper as it is a more reactive metal. Magnesium ribbon placed into copper sulfate solution will result in the formation of a magnesium sulfate solution and the depositing of copper metal. This results in a new salt being formed and the weaker metal coming out of solution and being deposited as a metal precipitate. Unreactive metals tend to stay umcombined. More reactive metals will displace (push out) less reactive metals from metal salt compounds. Iron (III) ions are reduced to become iron atoms Thermit Reaction 2Al (s) + Fe2O3 (s) Al2O3 (s)+ 2Fe (l) The more reactive the metal is, the more readily it forms compounds. Displacement Reactionsĭue to the relative reactivity of metals, given in the reactivity series, when combined they compete to form ionic compounds with other chemicals.
#ACTIVITY SERIES OF METALS SERIES#
The non-metal elements hydrogen and carbon have been included in the reactivity series as they are often used with metals in experiments and they are also used to help extract metals from their naturally occurring metal ores.

These have to undergo chemical processes to extract the pure metal from the ore. are found as copper ore, copper compounds. Gold, for example, can be found in nature as gold metal in rocks as it does not react readily. Metals at the top of the series tend to form ions more rapidly than those at the bottom. By comparing the rate at which each metal reacts with water and/or a dilute acid the metals can be placed in order to form the reactivity series. Metals readily react with water and dilute acids, during these reactions hydrogen gas is given off.
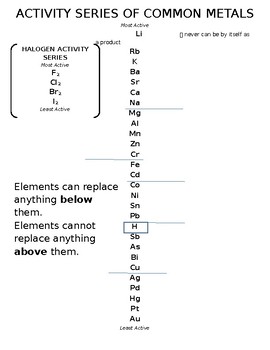
Metals can be ranked according to their level of reactivity to form the metal reactivity series. Some metals give up their electrons more readily than others and are, therefore, more reactive. Metal ions are positively charged as they lose negative electrons. This means that when they react they tend to lose electrons to form ionic compounds. Metal elements have either 1,2 or 3 electrons in their outer electron orbits.


 0 kommentar(er)
0 kommentar(er)
